TISSUE-GUARD Family

A Heritage of Healing
With more than 1.3 million units sold, our TISSUE-GUARD products offer clinical application across a broad range of indications including vascular, neurological, abdominal, thoracic and cardiothoracic surgery.1
Additional Product Benefits
Low Risk of Inflammatory Response
Through a proprietary manufacturing process, the intrinsic nature of the bovine pericardium material is maintained while meeting high levels of biocompatibility and demonstrating a low risk of inflammatory response.2,3
Strength, Durability and Excellent Handling
The TISSUE-GUARD products are similar to autologous tissue and are easy to handle and suture through; they resist suture line bleeding with excellent reapproximation.2,4,5
Multiple Size Options
The TISSUE-GUARD family of patches offer multiple size options and readily conform to multiplanar shapes.
Important Risk Information
DURA-GUARD
INDICATIONS FOR USE
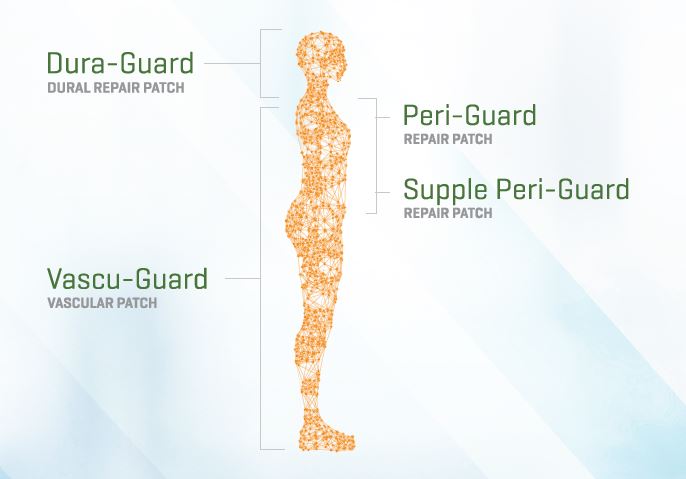
DURA-GUARD is intended for use as a dura substitute for the closure of dura mater during neurosurgery.
CONTRAINDICATIONS
DURA-GUARD is not designed, sold, or intended for use except as indicated.
Do not use DURA-GUARD in patients with a known sensitivity to bovine material.
ADVERSE REACTIONS AND WARNINGS
Failure to rinse the product may result in a sterile inflammatory reaction.
For safe and proper use of this device refer to the complete Instructions for Use.
CE 2797
PERI-GUARD
INDICATIONS FOR USE
PERI-GUARD is intended for use as a prosthesis for pericardial closure and soft tissue deficiencies which include defects of the thoracic wall, and intracardiac and great vessel repair.
CONTRAINDICATIONS
PERI-GUARD is not designed, sold, or intended for use except as indicated.
Do not use PERI-GUARD in patients with known sensitivity to bovine material.
ADVERSE REACTIONS AND WARNINGS
Failure to rinse the product may result in a sterile inflammatory reaction.
For safe and proper use of this device refer to the complete Instructions for Use.
CE 2797
SUPPLE PERI-GUARD
INDICATIONS FOR USE
SUPPLE PERI-GUARD is intended for use as a prosthesis for pericardial closure and soft tissue deficiencies which include: defects of the thoracic wall, and intracardiac and great vessel repair.
CONTRAINDICATIONS
SUPPLE PERI-GUARD is not designed, sold, or intended for use except as indicated.
Do not use SUPPLE PERI-GUARD on patients with a known sensitivity to bovine material.
ADVERSE REACTIONS AND WARNINGS
Failure to rinse the product may result in a sterile inflammatory reaction.
For safe and proper use of this device refer to the complete Instructions for Use.
CE 2797
VASCU-GUARD
INDICATIONS FOR USE
VASCU-GUARD is intended for use in peripheral vascular reconstruction including the carotid, renal, iliac, femoral, profunda and tibial blood vessels.
CONTRAINDICATIONS
VASCU-GUARD is not designed, sold, or intended for use except as indicated.
Do not use VASCU-GUARD in patients with known sensitivity to bovine material.
ADVERSE REACTIONS AND WARNINGS
Failure to rinse the product may result in a sterile inflammatory reaction.
For safe and proper use of this device refer to the complete Instructions for Use.
CE 2797